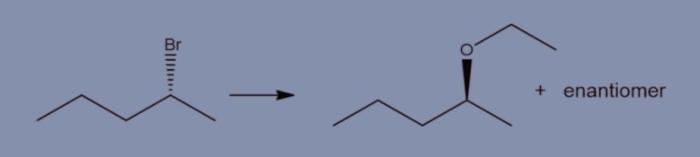
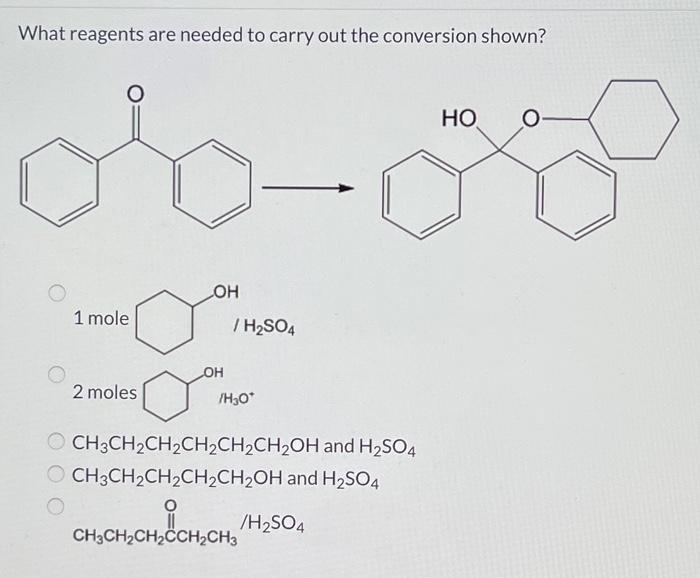
What reagents are suitable to carry out the conversion shown? This question lies at the heart of this discussion, where we embark on a journey to explore the intricate world of chemical conversions. By delving into the factors that govern reagent selection, we will uncover the key considerations for identifying the most appropriate reagents for a given conversion, ensuring optimal efficiency and selectivity.
The quest for suitable reagents begins with a thorough understanding of the chemical conversion process, its objectives, and the desired properties of the target molecule. Armed with this knowledge, we can then delve into the reaction mechanism, scrutinizing the intricate steps that lead to the desired transformation.
This mechanistic understanding serves as a guiding light in our search for reagents that can effectively orchestrate the desired chemical dance.
Chemical Conversion Process: What Reagents Are Suitable To Carry Out The Conversion Shown

The chemical conversion process involves the transformation of a starting material into a target molecule with desired properties. The target molecule is carefully designed to possess specific characteristics, such as enhanced stability, reactivity, or functionality.
The reaction mechanism Artikels the detailed steps of the conversion, including the breaking and formation of chemical bonds. Understanding the mechanism is crucial for optimizing the reaction conditions and achieving high product yields.
Reagent Selection Criteria
Selecting suitable reagents is essential for efficient chemical conversions. Key factors to consider include:
- Reactivity: Reagents should possess high reactivity towards the starting material to facilitate the desired reaction.
- Selectivity: Reagents should exhibit selectivity for the target molecule, minimizing side reactions and undesired products.
- Efficiency: Reagents should be cost-effective and minimize the use of hazardous or expensive chemicals.
Commonly used reagents include catalysts, oxidizing agents, reducing agents, and nucleophiles.
Reaction Conditions Optimization, What reagents are suitable to carry out the conversion shown
Optimizing reaction conditions is crucial for maximizing product yield and minimizing side reactions. Factors to consider include:
- Temperature: Temperature can affect reaction rates and product distribution.
- Pressure: Pressure can influence the equilibrium of reactions involving gases.
- Solvent: Solvent polarity can affect the solubility of reactants and products, as well as reaction rates.
- Catalysts: Catalysts can accelerate reaction rates without being consumed in the process.
Based on the selected reagents, specific guidelines for optimizing reaction conditions can be developed.
Alternative Reaction Pathways
Alternative reaction pathways may occur, leading to undesired products or reduced yield. Factors influencing selectivity include:
- Steric effects: Bulky groups can hinder certain reaction pathways.
- Electronic effects: Electron-withdrawing or electron-donating groups can alter the reactivity of functional groups.
- Solvent effects: Solvents can stabilize certain intermediates or transition states.
Strategies to minimize side reactions and improve product yield include using selective reagents, optimizing reaction conditions, and employing protecting groups.
Experimental Setup and Procedures
The experimental setup involves designing a reaction vessel and assembling the necessary equipment. Procedures include:
- Preparation of reagents: Reagents are prepared and purified as necessary.
- Reaction setup: Reactants, solvents, and catalysts are added to the reaction vessel.
- Reaction monitoring: The reaction is monitored using techniques such as TLC or spectroscopy.
- Product isolation: The product is isolated and purified using techniques such as filtration, extraction, or chromatography.
Safety considerations and waste disposal protocols must be followed throughout the process.
Product Characterization and Analysis
Product characterization involves verifying the identity and purity of the target molecule. Techniques used include:
- Spectroscopic techniques (NMR, IR, MS): These techniques provide information about the molecular structure and functional groups present.
- Elemental analysis: This technique determines the elemental composition of the product.
- Chromatographic techniques (HPLC, GC): These techniques separate and identify components of a mixture, including impurities.
Interpreting the results and confirming product identity is essential for ensuring the success of the chemical conversion process.
Helpful Answers
What is the most important factor to consider when selecting reagents?
Reactivity is the most crucial factor, as it determines the rate and efficiency of the reaction.
How can I improve the selectivity of a reaction?
Using reagents with high selectivity for the desired reaction pathway can minimize side reactions and enhance product yield.
What are some common techniques for optimizing reaction conditions?
Adjusting temperature, pressure, solvent, and catalysts can significantly impact reaction efficiency.